Background
The diagnosed age of de novo acute myeloid leukemia (AML) increased over the years, with a median current age of 68 years old. Elderly AML patients characterized by harboring high-risk mutations and significant comorbidities, usually display resistance to conventional chemotherapy, leading to poor outcomes. Although encouraging results from VIALE-A were reported in these years, over 30% participants had limited responses to the regimen of azacitidine + venetoclax after first-cycle induction. Thus, the selection of initial regimen for elderly AML is worth further exploration, especially for those with good fitness (the fitness was evaluated based on the physical ability, emotional status, cognitive function, and comorbidities). Here, we added idarubicin to azacitidine + venetoclax as the induction regimen (IAV regimen) for elderly fit-AML, and reported the preliminary results of efficacy and safety.
Methods
This single-center, single-arm, phase II trial (ChiCTR2300070190) was conducted at Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Shanghai, China). Elderly patients (aged 60-75 years) of newly diagnosed acute myeloid leukemia (AML) were assessed via modified comprehensive geriatric assessment (mCGA) for the fitness. From April 2023, 22 patients have been enrolled for the treatment of IAV regimen (cycle 1: IDA 6 mg/m 2 d1-3, AZA 75 mg/m 2 d1-7, VEN 100 mg d1, 200 mg d2, 400 mg d3-14; cycle 2: IDA 6 mg/m 2 d1-2, AZA 75 mg/m 2 d1-5, VEN 400 mg d1-14). Among them, 19 patients and 13 patients who have completed cycle 1 and cycle 2 treatment, respectively, were available for analyzing the preliminary efficacy and safety in the trial.
The primary objective of this study was to access the composite complete remission (cCR: CR + CRi) rate of IAV treatment. The secondary objective was to evaluate the MRD-negative rate, regimen-related toxicity, and safety. The duration of CR, overall survival rate and event-free survival rate would also be further accessed with sufficient follow-up time.
Results
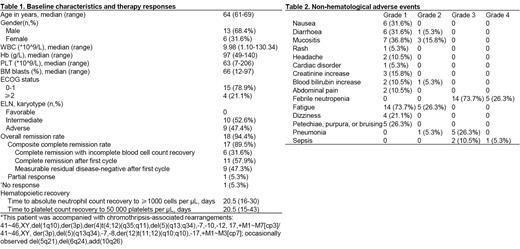
The median age of currently enrolled patients was 64 (61-69) years old, and males accounted for 68.4%. Detailed baseline characters were summarized in Table 1. Composite complete remission rate after 1-cycle IAV treatment was achieved in 89.5% (17/19) participants, among which 52.9% (9/17) got MRD-negative status. One patient (1/19, 5.26%) got partial remission, with 7.5% blasts remained. After cycle 2 treatment, the CR rate increased to 92.9% (13/14), and 92.3% (12/13) CR participants were MRD-negative (Table 1).
Till now, only 1 patient with chromothripsis-associated rearrangements showed no response and died on day 40 after treatment initiation. The median recovery time of neutrophils (ANC > 0.5×10 9/L) and platelet (PLT > 50×10 9/L) was 20.5 (16-30) days and 20.5 (15-43) days, respectively (Table 1). The most frequent grade 3/4 non-hematological adverse events (according to CTCAE version 5.0) were febrile neutropenia (grade 3, n = 14; grade 4, n = 5), pneumonia (grade 3, n = 5) and sepsis (grade 3, n = 2; grade 4, n = 1) (Table 2).
Conclusion
In this study, IAV regimen showed promising efficacy on elderly fit-AML patients, with high rates of MRD-negative remission. Non-hematological toxicities were tolerable under current observation. Based on the invigorating preliminary results, the efficacy and potential benefit of IAV regimen on elderly fit AML is worthy of follow-up in the future.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal